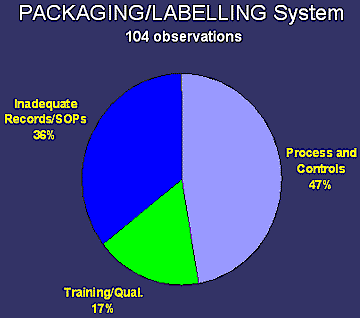
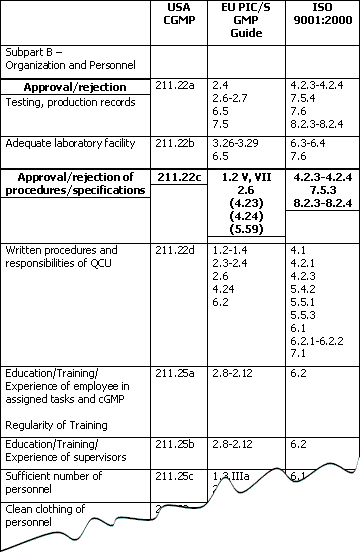
fda and eu gmp requirements
EU GMP Requirements Sterile medicinal product GMP Training ...
Staatliches Gewerbeaufsichtsamt Hannover. EU GMP Annex 1- Basic Elements. Clean Room Classification. ? US-FDA Requirements ? EU GMP Requirements .
http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2009/12/WC500017886.pdf
Good manufacturing practice - Wikipedia, the free encyclopedia
GMPs are enforced in the United States by the US FDA, under Section 501(B) of . The European Union's GMP (EU-GMP) enforces similar requirements to WHO .
http://en.wikipedia.org/wiki/Good_manufacturing_practice
Revision of FDA/EU GMP ISO Matrix - ECA Good Practice Guide
Jun 30, 2006 . The revised ECA Good Practice Guide is a comprehensive juxtaposition containing the requirements laid down in FDA's cGMP Guide, the EU .
http://www.gmp-compliance.org/eca_news_756.html
European Compliance Academy for GMP and Regulatory Affairs
Finally published: new EU GMP Chapter 1 with comprehensive Changes . the efficient implementation of FDA's cGMP requirements in routine operations.
http://www.gmp-compliance.org/
FDA/EU-GMP inspection in China - World Market - Sinopharm ...
FDA/EU-GMP inspection in China . Now the Chinese GMP regulations, which are being updated by SFDA, are almost same as those from European authority.
http://www.sino-pharm.com/en/worldMarket/inspectionInChina.html
FDA'S PHARMACEUTICAL GMP PROGRAMS ASSESSED BY EU
FDA'S PHARMACEUTICAL GMP PROGRAMS ASSESSED BY EU . City field offices to observe how the Agency sets standards and monitors and enforces the .
http://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm130390.htm
Changes Affecting EU GMP and The FDA's CGMP Non-Viable ...
What the revisions to ISO 14644 may mean to your cleanroom EU GMP and the FDA's cGMP set the target cleanliness levels for non-viable particles . In determining the required sample size ISO 14644 Annex B directs us to take a minimum .
http://www.cemag.us/article/changes-affecting-eu-gmp-and-fdas-cgmp-non-viable-particle-cleanliness-cleanrooms?page=0,2
FDA & EU Requirements for Documentation & Approval of GMP ...
Apr 23, 2011 . What does the FDA require for the documentation, signature, and approval of standard operating procedures (SOPs)? Are the requirements the .
http://gxpperspectives.com/2011/04/23/fda-requirements-for-documentation-approval/
FDA/EU cGMP Compliance for Laboratories
FDA/EU cGMP Compliance for Laboratories. Requirements and Tools for Implementation. Recorded. Order Button. When drug companies develop products .
http://www.labcompliance.com/seminars/audio200/default.aspx
New EU GMP Annex 11 on Computerized Systems Released
With strategies and tools for FDA and EU compliance. July 28 . Learn about specific requirements and get tools for implementation . Together with the updated EU GMP Chapter 4 on documentation it is the EU equivalent to FDA's Part 11.
http://www.labcompliance.com/solutions/expert_advice/part11-annex11/5101-annex11-update.aspx
Good Manufacturing Practice (GMP) | Regulations and Guidelines ...
Eudralex Volume 4 - GMP Human and Veterinary, EU GMP Guidelines for Human . FDA CBER Guidance/Guidelines/Points to Consider, FDA Guidances and .
http://www.barqa.com/committees-working-parties/good-manufacturing-practice/regulations-guidelines/
GLP vs GMP vs GCP
4) GLPs are not guidelines, they have the force of law. . 4 – EU Guidelines to GMP Medicinal products for human and . FDA GLP Regulations Apply to (58.1) .
http://www.pda.org/Chapters/North-America-cont/Southeast/Presentations/GLP-vs-GMP-vs-GCP.aspx
GMP Regulations/Guidelines - International Countries
GMP Regulations/Guidelines - International Countries. Pharmaceutical . http://ec .europa.eu/health/documents/eudralex/vol-4/index_en.htm. Annex 11 of EU GMP . Computerized . U.S. FDA - United States Food and Drug Administration .
http://www.labcompliance.com/info/links/international/international-gmp.htm
GMP and cGMP Particle Counting Requirements for EU and FDA ...
What is GMP? GMP stands for "Good Manufacturing Practices" as directed and regulated by FDA and EU agencies. Learn about viable and non-viable particle .
http://www.pmeasuring.com/pms/moreinfo/GMP
Changes Affecting EU GMP and The FDA's CGMP Non-Viable Particle
What the revisions to ISO 14644 may mean to your cleanroom EU GMP and the FDA's cGMP set the target cleanliness levels for non-viable particles in .
http://www.cemag.us/article/changes-affecting-eu-gmp-and-fdas-cgmp-non-viable-particle-cleanliness-cleanrooms